The 13th (2013) Yamazaki-Teiichi Prize Winner Biological Science and Technology
Irinotecan Hydrochloride : the First Established Anticancer Agent as Camptothecin Derivatives
| Winner | ||
|---|---|---|
| Tadashi Miyasaka | ||
| History | ||
| Mar. 1960 | Finished Pharmacology Course, University of Tokyo Graduate School of Chemistry | |
| Apr. 1960 | Teaching Assistant, Tokyo Medical and Dental University | |
| Mar. 1965 | PhD in Pharmacology, University of Tokyo Graduate School | |
| Oct. 1968 | Associate Professor, Showa University (Department of Pharmaceutical Chemistry, Faculty of Pharmacology) | |
| Apr. 1978 | Professor, Showa University (Chief Professor, Department of Pharmaceutical Chemistry, Faculty of Pharmacology) | |
| Apr. 1997 | Director, Faculty of Pharmacology, Showa University; Member of the Board of Directors, School Corporation Showa University | |
| Apr. 1999 | Professor emeritus, Showa University | |
| Apr. 2004 | Auditor, School Corporation Showa University | |
| Present | ||
| Winner | ||
|---|---|---|
| Seigo Sawada | ||
| History | ||
| Mar. 1978 | Finished the Doctor Course, Showa University Graduate School of Pharmacology | |
| Apr. 1978 | Researcher, Central Research Institute, Yakult Honsha Co., Ltd. | |
| Apr. 2002 | Chief Researcher, Yakult Honsha Co., Ltd. | |
| Apr. 2007 | Councilor, Central Research Institute, Yakult Honsha Co., Ltd. | |
| Oct. 2007 | Special Researcher, Yakult Honsha Co., Ltd. | |
| Present | ||
| Winner | ||
|---|---|---|
| Teruo Yokokura | ||
| History | ||
| Mar. 1969 | Finished the Master Course, University of Shizuoka Graduate School of Pharmacology | |
| Apr. 1969 | Researcher, Central Research Institute, Yakult Honsha Co., Ltd. | |
| Dec. 1973 | PhD in Pharmacology, University of Shizuoka Graduate School of Pharmacology | |
| Jun. 1999 | Member of Board of Directors and Central Research Institute Director, Yakult Honsha Co., Ltd. | |
| Jun. 2005 | Managing Director and Pharmaceutical Division Director, Yakult Honsha Co., Ltd. | |
| Jul. 2011 | Retirement from Yakult Honsha Co., Ltd. | |
| Present | ||
Reason for award
Overcoming cancers is one of the greatest topics of modern medicine. Since the 20th century, numerous studies have been conducted towards the goal of developing methods and drugs for cancer treatment. Dr. Tadashi Miyasaka worked together with Dr. Seigo Sawada, Dr. Teruo Yokokura and others to advance research & development, resulting in successful development of irinotecan hydrochloride, a drug effective on many types of cancer (colorectal cancer, gastric cancer, etc.). This drug has been approved in 100 countries, including Japan, France and USA, providing benefits to cancer patients across the world. The work by Dr. Miyasaka and his coworkers contributed not only to advances in R&D but also to creation of a 100 billion Yen-size market of an anti-cancer drug originating from Japan, playing a significant role in the treatment of many cancer patients. These researchers, judged to deserve the Yamazaki-Teiichi Prize, were thus elected as the awardees for this year.
Dr. Miyasaka and his-coworkers attempted to convert the chemical structure of camptothecin whose clinical use had been previously given up because of adverse reactions despite noteworthy anti-cancer activity. They synthesized many candidate compounds in a unique way and advanced preclinical and clinical (therapeutic) studies on some of these compounds while overcoming various difficulties (necessity of diverse biological studies, etc.), eventually leading to development of irinotecan hydrochloride. This is a pioneer case of successful development of a new drug conducted at the initiative of Japan in all stages (from the initial stage of development to the stage of market cultivation). Cerebrating their performance with this award will further stimulate and activate drug creation research in Japan from now on. The patent right on this compound has expired and there are now many generic versions of this drug on the market. This fact reflects the usefulness of this anti-cancer drug and the magnitude of the influence from the work achieved by these researchers.
Due to the aforesaid reasons, the three researchers, Dr. Miyasaka, Dr. Sawada, and Dr. Yokokura, will be awardees of the 13th Yamazaki-Teiichi Prize in biological science and technology.
Background of research and development
In 1966, M.E. Wall et al. investigated the anti-tumor activity of natural substances of various origins in the world within the framework of the cancer control campaigns in USA. A new substance isolated from a deciduous tall tree "Kiju" of Chinese origin (scientific name: Camptotheca acuminata Decsne) was named "camptothecin" by these researchers, and its chemical structure was determined.

Photo. 1 Kiju (Camptotheca acuminata Decsne)- leaf (left),
flower (upper right), seed (lower right)
Screening of the biological actions of camptothecin revealed anti-tumor activity in a mouse model of experimentally transplanted tumor. Following this finding, the National Cancer Institute (NCI), USA, conducted clinical trials on this substance in the first half of the 1970s. However, severe adverse reactions such as bone marrow suppression and hemorrhagic cystitis were induced by this substance, and its development as a drug for treatment was given up. For preclinical and clinical studies, it is essential to conduct sufficient evaluation with scientific methods concerning the optimum substances selected from numerous candidate compounds. If camptothecin of a natural origin can be used as the stating material, it will be possible to convert this material into a new compound by one step of a new chemical reaction. This approach will be much more efficient than the approach of full synthesis involving many steps and is expected to yield more samples for studies.
Achievements
At present, well-designed preclinical studies are needed before a clinical trial is started. We considered that to propose a new structure which can satisfy such requirements, we should conduct detailed chemical characterization of a given material and attempt its chemical conversion in a radial manner while concomitantly assessing the physiological characteristic in animal studies. Of the possible structures of camptothecin made of condensation of 5 rings, i.e. quinolone (A,B ring), pyrroline (C ring), α-pyridone (D ring) and δ-lactone (E ring) (Fig. 1(1)), we selected a three-dimensionally unique structure. That is, we devised a strategy to introduce a substituent with the following features: (1) possessing asymmetric carbon atom, (2) leaving E and D rings with polarized functional groups unchanged as far as possible, and (3) preserving the two-dimensional structure of the C-H bond of non-polarizing A, B and C rings which assume an approximately two-dimensional structure. Survival rate of L1210-carrying mice served as an indicator of the activity of the compounds thus designed. In evaluation using whole animals, the quantity of the sample needed is at least 100 mg. This imposed large burdens on the chemical synthesis staff but the thus performed assessment yield quite precise information.
Chemical conversion of camptothecin, which is little soluble in water and organic solvents, was quite difficult. We paid attention to the fact that this substance, insoluble in dilute HCl, was highly soluble in concentrated sulfuric acid, while forming a salt. We thus obtained amino form, hydroxyl form, alcoxy form, halogeno form, etc. of this compound through radical alkylation in concentrated sulfuric acid (reaction with fatty alcohol or aldehyde as a radical source in the presence of Fenton's reagent for oxidation: e.g., alkylation with one carbon atom decomposition), nitration at 9-position or 12-position of A ring with sulfuric acid or nitric acid, nitration (11-position) after converting A ring into aniline derivative through B ring catalytic reduction, or nitration (10-position) after conversion into acetanilide derivative. The 10-hydroxyl form had previously been isolated also from natural materials and had been evaluated to be low in toxicity, but information on this form was not sufficient enough to encourage studies aimed at clinical use. 10-hydroxycamptothecin was obtained with a high yield also through camptothecin-N-oxide photochemical reaction. This method is valid also as an industrial method and has been incorporated into the irinotecan production processes. As the most excellent form, 7-ethyl-10-hydroxycamptothecin (SN-38, Fig. 1(2)) was selected, and an attempt was made to make this compound soluble in water via the 10-position hydroxyl group. Of the many compounds formed in this way, the hydrochloride salt of irinotecan composed of piperidinopiperidine group bound via the urethane bond is a compound characterized by high stability in the form of aqueous solution and physical properties suitable for drip infusion (Fig. 1(3)).
The international generic name adopted for this compound is Irinotecan Hydrochloride Trihydrate.
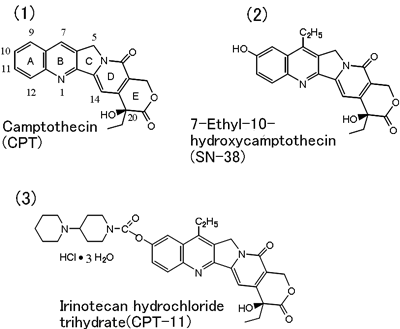
Fig. 1 Chemical conversion of camptothecin into irinotecan hydrochloride
Meaning of the achievements
Captothecin derivatives were synthesized on the basis of the findings from basic studies of heterocyclic chemistry. Their anti-tumor activity was evaluated, with tumor-carrying mouse survival rate serving as an indicator. In this way, the structure-activity correlation of camptothecin derivatives was established. The most excellent compound 7-ethyl-10-hydroxycamptothecin (SN-38) was selected, subsequently yielding irinotecan hydrochloride (CPT-11), a compound linked to a water-soluble functional group (piperidinopiperidine hydrochloride) via the urethane bond at 10-position hydroxyl group. After many preclinical studies, clinical trials on this compound were carried out on the basis of scientific evidence. The compound was approved by the regulatory authority in Japan (1994), France (1995) and USA (1996). By 2002, it was approved in 100 countries. This drug exerts activity against gastrointestinal cancer, lung cancer, gynecologic cancer, etc., through inhibiting DNA topoisomerase I, an action mechanism totally differing from that of conventional metabolic antagonists such as 5FU. Particularly in the treatment of colorectal cancer, this drug has been playing an important role as the first-line drug for uncombined drug therapy and combined drug therapy. Although 17 years were taken from the start of research to approval of this drug, this drug is appraised highly in both healthcare and academic standpoints still at present (19 years after its approval). Because of its novel action mechanism, this drug exerts synergistic effects when used in combination with other drugs, thus contributing greatly to improving the mean survival rate of patients.
FOLFIRI therapy is a regimen of combined drug therapy primarily involving folic acid derivative, 5FU and irinotecan. Mean survival rate with this therapy has reached 25 months.
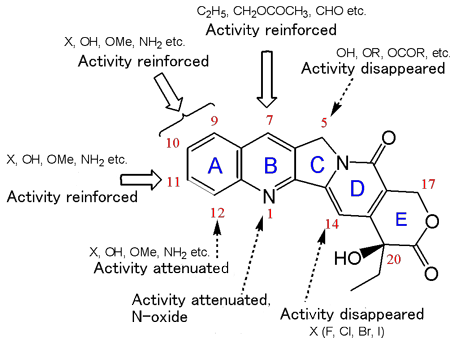
Fig. 2 Structure-activity correlation
