The 19th (2019) Yamazaki-Teiichi Prize Winner Biological Science and Technology
Revolution in Molecular Targeted Therapies for Cancer and Realization of Genomic Medicine
| Winner | ||
|---|---|---|
| Hiroyuki Mano | ||
| History | ||
| Mar. 1984 | Graduated from Faculty of Medicine, The University of Tokyo | |
| Aug. 1991 | Assistant Professor, Third Department of Internal Medicine, Faculty of Medicine, The University of Tokyo | |
| Jun. 2001 | Professor, Division of Functional Genomics, Jichi Medical University | |
| Apr. 2013 | Professor, Graduate School of Medicine, Faculty of Medicine, The University of Tokyo | |
| Apr. 2016 | Director, Research Institute, National Cancer Center (Present) | |
| Jun. 2018 | Director, Center for Cancer Genomics and Advanced Therapeutics, National Cancer Center (Present) | |
Reason for award
Dr. Mano discovered EML4-ALK, a new lung cancer oncogene, and demonstrated that fusion-type oncogenes derived from chromosomal translocation are found even in epithelial tumor for the first time in the world. Subsequently, he developed the procedure for clinical diagnosis of EML4-ALK mutation and contributed to the world¡Çs fastest approval of an ALK inhibitor crizotinib. In addition, he was the first to identify crizotinib-resistant mutations within EML4-ALK. This led to the development of the second-generation ALK inhibitors such as alectinib. Alectinib, which is already approved in Japan and known as the most effective anticancer agent, has shown a magical response rate of 94%. Furthermore, the winner proposed the cancers with abnormal activation of ALK be collectively called ¡ÈALKoma¡É, a beyond-organ, gene-based cancer classification scheme that has led the ¡Ècancer genomic medicine¡É. Thus, the winner has driven the paradigm shift in global cancer research and medical treatment. His achievements cover a broad range of areas from basic to clinical applications. Particularly, it is worth acknowledging that his remarkable research achievements have actually saved lives of tens of thousands of cancer patients around the world.
Considering all his achievements, Dr. Mano has been awarded the 19th Yamazaki-Teiichi Prize in Biological Science and Technology.
Background of research and development
The number of cancer deaths is 8.2 million worldwide every year, and the number of young patients is increasing in various cancer types such as lung cancer. Overcoming cancer is one of the greatest desires of human beings, but there were few effective anticancer drugs for patients who cannot be completely cured by surgical treatments. To overcome these situations, many ¡Èmolecular-targeted agents¡É, targeting functional molecules that are important for growth of cells, have been developed. Unfortunately, there were few drugs that could improve the prognosis of patients in mono therapies at the end of the 20th century.
The winner hypothesized that almost all molecular-targeted agents were ineffective because their target proteins were not essential for proliferation of cancer cells. He anticipated that there are probably a few intrinsic oncogenic proteins in each type of cancer and that if the agents selectively target such proteins, they would become the magic bullets. If this hypothesis is correct, identification of the essential growth drivers in each cancer subtype should be the most important element for the development of effective molecular-targeted therapeutics.
Achievements
The winner, therefore, has developed a novel and original technique that enables identification of essential oncogenes. In cells, messenger RNAs (mRNAs) are made from genome, and translated into proteins that finally exert their functions. In cancer cells, mRNAs from essential oncogenes should have been made. Therefore, mRNAs were extracted from clinical cancer specimens, converted to complementary DNAs (cDNAs), and incorporated into retroviruses to build a retroviral cDNA expression library. When mouse 3T3 fibroblast cells were infected with this recombinant virus library, the shape of the cells to which the oncogenes were introduced becomes abnormal and can be detected.
Application of this technique to a lung cancer specimen led to the discovery of a novel oncogene EML4-ALK (Fig. 1).
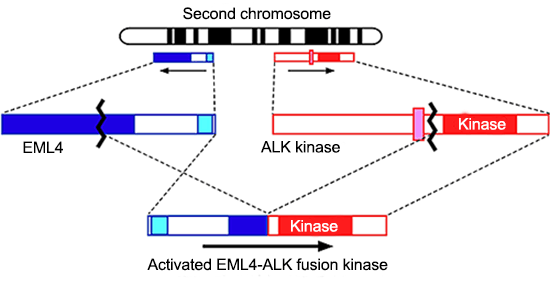
Fig.1¡¡Discovery of EML4-ALK fusion gene
The EML4 gene and ALK gene are aligned closely to each other in the opposite direction within the second chromosome in human normal cells. However, in some lung cancer cells, an intrachromosomal inversion involving the two genes takes place to generate the EML4-ALK fusion gene. Previously, such fusion oncogenes generated through chromosomal structural abnormalities were believed to be limited to hematological malignancies. This discovery of EML4-ALK, however, directly demonstrated that such notion was incorrect and even lung cancer, a representative solid tumor, is caused by a chromosomal rearrangement. Normal ALK protein is an enzyme (kinase) that promotes cell growth. As a result of chromosomal translocation, the ALK protein becomes fused with EML4, leading to a drastic increase in the enzyme activity and an oncogenic protein. In fact, when mice expressing EML4-ALK in the lung were generated, hundreds of lung cancer nodules were developed immediately after birth. However, such nodules promptly disappeared after administration of an ALK inhibitor (Fig. 2).
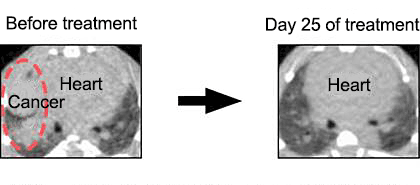
Fig. 2¡¡Therapeutic experiment of mice expressing EML4-ALK
Based on this discovery, many ALK inhibitors have been investigated in clinical studies. Crizotinib, which was the first investigated drug, demonstrated the response rate of approximately 60% in phase Ⅰ/Ⅱ studies. This remarkable therapeutic effect prompted FDA, the US drug approval agency, to approve crizotinib without requiring large-scale phase Ⅲ clinical trials. This approval, which was granted only four years after the discovery of EML4-ALK, was the fastest in the history of the development of anticancer drugs in the world.
Furthermore, the winner also elucidated the mechanism by which EML4-ALK-positive lung cancer acquires resistance to crizotinib. Based on the obtained findings, many pharmaceutical companies developed “second-generation ALK inhibitors” less vulnerable to resistance. Among them, alectinib demonstrated a miraculous response rate of 93.5% in phase Ⅰ/Ⅱ clinical studies and was approved as a therapeutic agent for lung cancer by the Ministry of Health, Labour and Welfare in July 2014.
The discovery of EML4-ALK has, thus, provided a magic bullet against lung cancer harboring this gene, and it further led the cancer genomic medicine at the same time. When ALK becomes fused with EML4, lung adenocarcinoma is induced. ALK was also shown to induce lung cancer when fused with KIF5B. In addition, it was demonstrated that ALK fused with NPMI induces malignant lymphoma, with VCL inducing renal cancer, and with TPM3⁄4 induces sarcoma. In other words, single ALK gene becomes activated to generate cancers in multiple organs. The winner proposed that all cancers caused by this activated ALK should be called ¡ÈALKoma.¡É In other words, he proposed a classification method for cancer based on oncogene and not the organs. The winner also succeeded in discovering ROS1 and RET fusion oncogenes in lung adenocarcinoma. This reveals that the lung adenocarcinoma is not a homogeneous entity but an aggregate consisting of small groups of cancer caused by the fusion of ALK⁄ROS1⁄RET genes.
Meaning of the achievements
As a result of a series of achievements by the winner, regardless of the organs from which the cancer is derived, it can be said that the most suitable drug cannot be selected for a given patient unless genomic profiles are fully examined. This is precisely ¡Ècancer genomic medicine.¡É In this way, the world has entered an era where a therapeutic strategy is determined based on cancer multi-gene panel testing where abnormal sequences of multiple cancer-related genes can be analyzed at once.
In Japan, it is necessary to build a cancer genomic medicine system as soon as possible. In 2018, the Ministry of Health, Labour and Welfare established the ¡ÈCenter for Cancer Genomics and Advanced Therapeutics¡É which consolidates the genomic and clinical information of patients on cancer genomic medicine. The winner is now engaged in the promotion of cancer genomic medicine under the national health insurance system as the first director of the Center.
