The 18th (2018) Yamazaki-Teiichi Prize Winner Material
Design and Realization of Lithium Ionic Conductors and All-Solid-State Batteries
| Winner | ||
|---|---|---|
| Ryoji Kanno | ||
| History | ||
| Mar. 1978 | Graduated from Department of Chemistry, School of Science, Osaka University | |
| Mar. 1980 | Completed the First Semester of Doctoral Course, Department of Chemistry, School of Science, Osaka University | |
| Apr. 1980 | Research Associate, Chemistry for Resources, Faculty of Engineering, Mie University | |
| Oct. 1989 | Associate professor, Department of Chemistry, Faculty of Science, Kobe University | |
| Apr. 2001 | Professor, Interdisciplinary Graduate School of Science and Engineering, Tokyo Institute of Technology | |
| Mar. 2018 | Professor, Institute of Innovative Research, Tokyo Institute of Technology | |
| Present | ||
Reason for award
A conventional lithium-ion battery contains a positive and a negative electrode immersed in an electrolytic solution. The need for the solution itself came from the fact that there were no known solid electrolytes with greater capability to transfer ions than electrolytic solutions.
Ryoji Kanno has been researching and developing solid electrolytes for many years and had discovered in 2011 that Lithium Superionic Conductor, Li10GeP2S12 (LGPS), exhibits higher conductivity (higher ion conductivity) than an organic electrolyte currently used in practical applications. This was not only a breakthrough in solid electrolyte research conducted for over half a century but also had a strong impact on secondary battery-related industries. Kanno also discovered that Li9.54Si1.74P1.44S11.7Cl0.3 has better properties than LGPS. Based on this, in collaboration with a company, he developed an all-solid-state battery with higher energy density than existing lithium-ion batteries and discharge at large currents. Furthermore, he clarified its mechanism. As all-solid-state batteries are now extremely promising candidates for safe next-generation energy storage devices, their development as industrial materials has been conducted globally.
Thus, all solid-state batteries can be expected to contribute to society in the near future.
Based on the above considerations, Dr. Kanno has been awarded the 18th Yamazaki-Teiichi Prize in Material.
Background of research and development
New secondary batteries that meet the needs of the forthcoming electrified society are eagerly awaited. These batteries will be used as a power source for electric vehicles, robots, drones and so forth and their performance will exceed that of lithium-ion batteries. Various next-generation secondary battery devices have been researched and developed. Among them, attempts to solidify batteries themselves, that is, to develop all-solid-state batteries, were made early on but adequate performance that would meet society¡Çs demand has long eluded us.
Solids, namely so-called superionic conductors, are used as electrolytes in all-solid-state batteries. Special emphasis has been placed on the search for lithium superionic conductors in order to achieve the specific, tangible goal of remarkably improving the safety of secondary batteries. These have widely been in use despite potentially hazardous combustible organic solvents acting as electrolytes. Many candidate substances have been discovered since the 1960s, but the ionic conductivity of all these solid substances was far lower than those of liquids. As a result, studies on the fabrication of devices remained obviously limited.
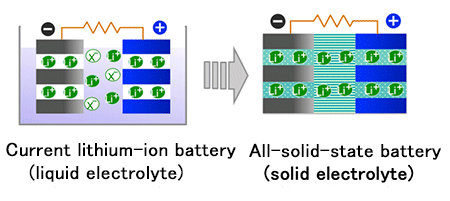
Fig. 1¡¡Development from lithium ion batteries to all-solid-state batteries
Achievements
The prizewinner has long been involved in the development of superionic conductors, solids that exhibit high ionic diffusion, and created a revolutionary lithium superionic conductor, Li10GeP2S12 (referred to as LGPS) and related substances, with ionic conductivity exceeding that of a liquid despite being a solid. Furthermore, he demonstrated that all-solid-state batteries manufactured with this new substance as electrolyte have charge and discharge properties that highly exceed both lithium-ion batteries with high energy densities and capacitors with high output properties, even at the initial stage of fabrication. The discovery of this new superionic conductor disposed of prior common knowledge that solids could not compete with liquids; it was not only a breakthrough in solid electrolyte research but also had a strong knock-on impact on battery-related industries. Not only is this a significant achievement, one that should be regarded as the ultimate goal of a prizewinner who has conducted research for more than 30 years to discover new high ionic conductivity materials, but it also opens up entirely new possibilities for secondary batteries. Figure 2 shows the temperature dependence of ionic conductivity. This indicates that ionic conductivity of LGPS exceeds that of electrolytic solutions. The emergence of such solid electrolytes was unexpected and the discovery of inorganic ionic conductors has affected all fields of materials science leading to renewed exploratory research not only in inorganic materials but also in polymers, liquids and so on. Furthermore, he analyzed the structure of a series of compounds and demonstrated that lithium ions are continuously distributed, a structure characteristic of high ionic conductivity.
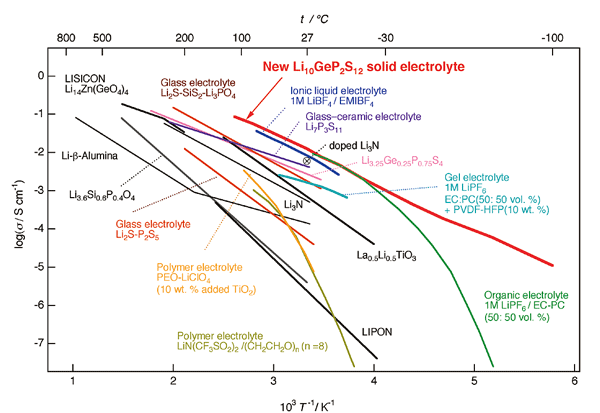
Fig. 2¡¡Temperature dependence of ionic conductivity of LGPS reported in 2011 (red) together with ionic conductivity of a liquid electrolyte (green)
Properties of all-solid-state batteries using the new materials far exceed those of lithium-ion batteries. Figure 3 shows an example of so-called “Ragone” plot measuring actual performance for various electric storage devices. Figure 3 displays the properties of each device with respect to output density and energy density for the device. For example, the capacitor has high output power, the lithium-air battery has high energy density and the lithium battery has both. It is obvious that the output properties of the all-solid-state batteries developed by the prizewinner exceed those of lithium-ion batteries, and in fact almost reach the level of capacitors. A conventional claim is that the key benefit from solidification of secondary batteries is high reliability without risk of combustion and explosion. In addition to these properties, it is a proven fact that high output power and high charging speed are intrinsic benefits of solid-state batteries. These properties overcome intrinsic limitations in lithium-ion batteries using organic electrolyte solutions with low ionic conductivity and have the potential to change the morphology of current secondary batteries themselves.
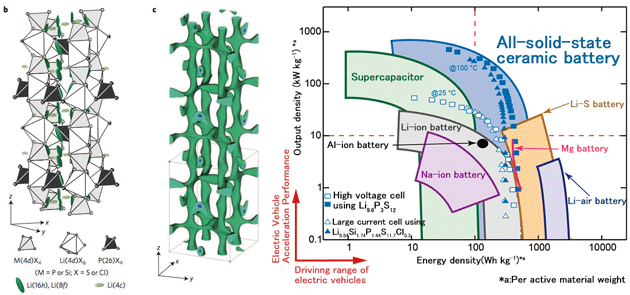
Fig. 3¡¡Performance of an electric storage device (Ragone plot) (from Nature Energy, 2016). This shows that output properties and energy densities of all-solid-state batteries exceed those of existing lithium-ion batteries.
Meaning of the achievements
As described above, all-solid-state batteries manufactured using materials described above have such properties as an energy density that is higher than existing lithium-ion batteries and an ability to discharge at larger current than capacitors. In addition, it became apparent that these excellent properties are attributable to the super-fast diffusion of lithium enabled by solid electrolytes. The most promising candidate for next-generation electric storage devices has thus emerged. Immediately after the announcement of this achievement, mainstream domestic newspapers as well as over 20 overseas media organizations provided coverage. Subsequent coverage could be found in business papers, business magazines, and scientific and economic pages of general newspapers. The race for the development of all-solid-state electric storage devices is now spreading globally. The ability of existing lithium-ion batteries to meet the needs of the forthcoming electrified society has been questioned; specifically their ability to act as a power source for electric vehicles and grid connection, robots or drones. This objective may be secured instead by developing all-solid-state batteries with a performance far exceeding that of existing lithium-ion technology. A national project to develop all-solid-state batteries has been initiated and an intense development race among domestic and overseas companies has already started.
In order to deploy solid batteries to practical applications, adjustments to existing manufacturing processes involving lithium-ion batteries and liquid batteries are necessary as solid batteries point to an entirely new technological framework. Following the development of technology associated with the process of manufacturing each component of batteries, work on battery assembly has been carried out. The discovery of new materials by the prizewinner and subsequent research on elucidating intrinsic properties of solid batteries has undoubtedly lead to the development of practical batteries.
