The 11th (2011) Yamazaki-Teiichi Prize Winner Biological Science and Technology
Development of a crystallization method utilizing phospholipids and elucidation of the mechanism of calcium pump
| Winner | ||
|---|---|---|
| Chikashi Toyoshima | ||
| History | ||
| Mar. 1978 | Graduated Department of Physics , Faculty of Science, the University of Tokyo | |
| Mar. 1983 | Completed Doctor's Course, Department of Physics, Graduate School of Science, the University of Tokyo | |
| Apr. 1988 | Researcher, MRC Laboratory of Molecular Biology (UK) | |
| Jul. 1989 | Researcher, Frontier Research Program, Institute of Physical and Chemical Research International | |
| Jan. 1990 | Assistant Professor, Faculty of Science (Faculty of Bioscience and Biotechnology), Tokyo Institute of Technology | |
| May 1994 | Professor, Institute of Molecular and Cellular Biosciences, the University of Tokyo | |
| Present | ||
Reason for award
Chikashi Toyoshima has conducted structural analyses of a protein called calcium pump, which is essential to biological activity, almost all by himself. The protein transports calcium ions across biological membrane. He elucidated the atomic structures of the protein in all 4 principal states that constitute the reaction cycle, and determined 8 crystal structures in total including its intermediate (transition) states. This is the first in the world that the path of the structural change of a protein, including water soluble and membrane proteins, has been shown in such details.
The key to success was to stabilize the intermediates by controlling the state of the membrane protein precisely during crystallization. His success in determining even the intermediate structures, with various ideas free from the established methodology, for example, addition of phospholipid contrary to common practice, delicate control of conditions and sample compositions throughout the crystallization process, encourages and provides driving force for following researchers, and contributes to the development of calcium pump research based on his results.
Many important physiological functions are conducted by membrane proteins, which are important targets of drug discovery as well. As analyses of their functions and structures progressed only very slowly, the achievement by Toyoshima who solved the most difficult problem has been recognized internationally. With Toyoshima's research results and research methods, we can expect further great and rapid development in this field in near future. The elucidation of the atomic structures of all 4 principal states and the determination of the 8 crystal structures in total including their intermediate (transition) states may lead to future drug development for each intermediate, posing major impact on the whole drug development research.
For the above reason, Chikashi Toyoshima shall be awarded the 11th Yamazaki-Teiichi Prize in Biological Science and Technology.
Background of research and development
Three-dimensional crystallization of a membrane protein is famous for its difficulty, and is represented as one type of a high-risk, high-return study.
The first crystallization was accomplished for the reaction centers of photosynthetic bacteria by H. Michel from Germany, who was awarded Nobel Prize in Chemistry for his achievements in 1988.
Michel advocated that it would be necessary to prepare a crystal with packing completely different from in vivo by removing phospholipid (Type 2 Crystal, Fig. 1) for crystal analysis of membrane proteins solubilized with a surfactant, and crystals in which proteins naturally buried in a lipid bi-layer membrane are stacked (Type 1 Crystal) might not be good since they would not grow. He also stated that phospholipid might be originally inhomogeneous posing adverse influence on crystallization. His assertion was believed broadly and, so to speak, became a common sense.
Since steam diffusion method was exclusively used for three-dimensional crystallization at the X-ray level, and a method of removing surfactant by dialysis forming lipid bi-layer membrane (two-dimensional crystal) was used for crystallization at the electron microscope level, it was believed that "Crystallization at the X-ray and electron microscope levels are essentially different".
Ca2+ pump protein (SERCA1a) in skeletal muscle is naturally present in great quantity, and its purification method had been already established in 1970. Therefore, it's one of few membrane proteins which anyone can crystallize, so to speak, and rumors that crystals were made appeared and disappeared for over 20 years or more duration. Although crystals were obtained at the electron microscope level, no one could remove phospholipid without losing activity or make crystals as large as the X-ray level.
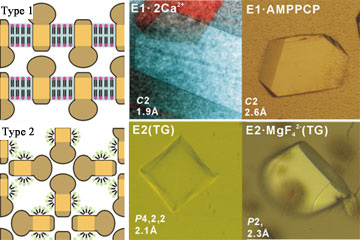
Fig. 1 Packing of three-dimensional crystals of membrane proteins and
three-dimensional crystals of Ca2+ pump proteins in various states
Achievements
It was in the middle of the 1980s that Toyoshima started to deal with Ca2+ pump proteins. At first, a reconstruction method of three-dimensional image of a tube-like crystal by an electron microscope was developed, and the first conformation of uncombined Ca2+ was obtained. On the other hand, it succeeded in production of a three-dimensional ultra-thin microcrystalline showing good electron diffraction pattern under Ca2+ existence, however, no crystal large enough for X-ray crystal structure analysis could be obtained. However, considering that there might not be essential difference between crystallization at the X-ray and electron microscope levels, Toyoshima decided to investigate extension of the electron microscope level method. Actually, addition of phospholipid was essential to maintain activity during purification in the case of Ca2+ pump proteins, and the difference between the aforementioned 2 methods might be only "whether to use a precipitant effectively," was what he considered.
Such trial contrary to the common sense resulted in success in determination of the Ca2+ pump crystal structure at the resolution of 2.6 Å which was high resolution as a membrane protein crystal at that time. It was in 2000. This was the first structure determination in a metal ion pump, moreover, Ca2+ pump which is the ion most extensively used in biocontrol, thus, it had a very great impact. Furthermore, Toyoshima succeeded in structure determination of 8 intermediate states almost completely covering the reaction cycle of Ca2+ pump by using crystallization method using this phospholipid and a metal fluoride complex as an analogue of phosphoric acid (Figs. 1, 2). The result enabled us to understand the outline of the mechanism transporting ions against the concentration gradient from the atomic structure. The magnitude of the structural change revealed by the result which nobody could anticipate, greatly reversed the conventional common sense, for example the large slide movement between transmembrane helice that was believed to be engaged firmly. Furthermore, it was found that the ion pump uses a lipid bi-layer membrane as a part of transportation mechanism, shedding a new light on the physiological role of lipid bi-layer membrane.
Although this crystallization method was the expansion of the two-dimensional crystallization method for an electron microscope, the application to three-dimensional crystallization was not easy. Since there are many factors in crystallization, success will be achieved only after they are set properly. To begin with, it was not even known what factor was an important factor or not. For example, the type of phospholipid (single chain or mixture, percentage of saturation and unsaturated fatty acid, etc.), the quantity ratio of protein: phospholipid: surfactant are important factors which may affect the success of crystallization, thus, systematic investigation was required. Therefore, many ideas for improving repeatability were necessary. In particular, establishment of a quick and simple quantitative method of phospholipid and a surfactant was important.
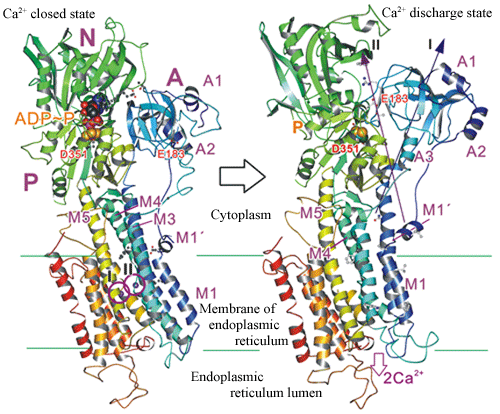
Fig. 2 Crystal structure of Ca2+ pump corresponding to states of immediately before and after releasing two Ca2+ (I, II) confined in he membrane. A domain rotates around the axis I by 90 degrees. A ribbon indicates an alpha helix and an arrow indicates a beta sheet.
Meaning of the achievements
With the success of this study, other groups came to succeed in crystallization by actively adding phospholipid, and greatly contributed to the expansion of the structural analysis of membrane proteins. Especially, it is very meaningful that it was proved that the structural analysis of membrane proteins originally functioning in a lipid bi-layer membrane may be possible under almost natural condition. Additionally, up to date, there are few proteins including water-soluble ones whose reaction mechanism have been structurally understood in such details.
The design of a drug based on the crystal structure with high resolution may reduce trial and error remarkably, enabling great reduction of time and cost needed for development. It has been said that most targets of future drug design are membrane proteins, especially, structure study of membrane proteins of higher animals is important with respect to medicine and drug design. On the other hand, in the current situation of searching for those can be crystallized by the conventional method and performing structure determination, most of them are from bacteria even if they are related proteins, structure determination of higher animal membrane proteins is quite rare. Active addition of phospholipid may greatly expand the range of membrane proteins which can be crystallized. Therefore, the importance will increase remarkably in the future. Actually, many cases in which crystallization succeeded for the first time by actively introducing phospholipid are being reported.
