The 8th (2008) Yamazaki-Teiichi Prize Winner Material
Development of New Olefin Polymerization Catalysts and Their Applications to Novel Polyolefinic Materials
| Winner | ||
|---|---|---|
| Terunori Fujita | ||
| History | ||
| Mar. 1982 | M.S., Hokkaido Univ. Graduate School of Science | |
| Apr. 1982 | Entered Mitsui Petrochemical Industries, Ltd. (currently Mitsui Chemicals, Inc.) | |
| Dec. 1988 | Ph.D. (The Louis Pasteur Univ. in Strasbourg, France) | |
| Jul. 2001 | Research Fellow (to Mar. 2008) | |
| Jul. 2005 | Catalysis Science Laboratory, General Manager¡¡(incumbent) | |
| Apr. 2008 | Executive Officer | |
| Present | ||
| Winner | ||
|---|---|---|
| Makoto Mitani | ||
| History | ||
| Mar. 1989 | M.S., Kyoto University, Faculty of Engineering, Industrial Chemistry | |
| Apr. 1989 | Entered Mitsui Petrochemical Industries, Ltd.(currently Mitsui Chemicals, Inc.) | |
| Mar. 1997 | Doctor of Engineering, Kyoto University, Graduate School of Engineering | |
| Jul. 2005 | Catalysis Science Laboratory, Polymerization Catalysis Unit, Leader | |
| Present | ||
Reason for award
Olefin-based polymers, as represented by PE and PP, are modern indispensable materials. They are used widely in everything from food packaging and kitchenware to gasoline tanks and automotive parts. Polyolefins are useful because they are safe, inexpensive, and rich in material properties.
The accomplishments upon which the awarding of this prize is based are (1) the development of a new phenoxy-imine complex catalyst with revolutionary performance for olefin-based polymer synthesis, and (2) the resultant development of new, high performance, value-added polyolefinic materials.
Historically, polyolefins came to be synthesized by means of the Ziegler-Natta catalysts developed in the 1950s. This type of catalyst, for which the 1963 Nobel Prize in Chemistry was awarded, is a heterogeneous catalyst represented by Al (C2H5) 3-TiCl4,. Thereafter, catalyst performance gradually improved through the advent of homogeneous group 4 metallocene catalysts. The phenoxy-imine complex catalyst (FI catalyst) is a heteroatom [O-,N] coordinated, group 4 transition metal olefin polymerization catalyst (or post-metallocene catalyst). Its ethylene polymerization activity surpasses that of group 4 metallocenes and it possesses versatility vis-a-vis cocatalyst selection. The FI catalyst has made possible the development of polyolefinic materials that were difficult or impossible to synthesize with conventional catalysts. These results are based on a ligand oriented catalyst design concept in which the fundamentals of catalyst design were reconsidered; instead of focusing on metals attention was directed toward ligands that conventionally had played only an ancillary role. Therefore, attention was focused on the electronically flexible properties of ligands with a non-symmetric structure and a small HOMO-LUMO energy gap¡¡The spillover effect of the FI catalyst development has been immense. It includes the synthesis of linear PEs with absolutely no branching (high ethylene selectivity), selective vinyl- or Al-terminated PEs and polyolefinic block copolymers (precise control over chain transfer reactions), and ultra-fine non-coherent PE particles (versatility vis-a-vis cocatalyst selection), and it also includes the possibility of ethylene/polar monomer copolymerization.¡¡Thus it is that the Eighth Yamazaki-Teiichi Prize in Materials has been awarded to Messrs. Terunori Fujita and Makoto Mitani for their ¡ÈDevelopment of New Olefin Polymerization Catalysts and Their Applications to Novel Polyolefinic Materials.¡É
Background of research and development
Polyolefinic materials, such as polyethylene (PE) and polypropylene (PP), are not only low density and inexpensive but are also useful materials possessing excellent properties and processability. At present, most of these materials are manufactured using heterogeneous Ziegler-Natta catalysts and group 4 metallocene catalysts (Kaminsky catalysts). In recent years, the properties and functions required for polyolefinic materials have become more sophisticated and diverse, necessitating the development of new high-performance catalysts that can produce high-performance and value-added polyolefinic materials.
Achievements
In contrast to conventional concepts regarding catalyst development that emphasize metals, Dr. Fujita et al. have carried out research based on a ¡Èligand oriented catalyst design concept¡É, a concept that is founded on the belief that the flexible electronic nature of a ligand is a key requirement for achieving high activity. Such an approach has resulted in the discovery of bis (phenoxy-imine) early transition metal complexes (now known as FI catalysts) that demonstrate extremely high ethylene polymerization activity (Fig. 1). The ethylene polymerization activity of FI catalysts surpasses that of group 4 metallocene catalysts. The catalyst turnover frequency (TOF) reached a maximum of 65,000/sec/atm (reacting 65,000 ethylene molecules per second under atmospheric pressure conditions). This TOF is a new record not only for olefin polymerization, but for any catalytic reaction known at present, including oxidation, reduction and alkylation reactions.
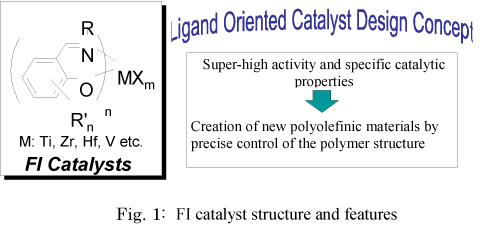
In addition to high activity, FI catalysts have the following five features : (1) They allow for a wide variety of catalyst design possibilities (diversity of structure) , (2) they exist as mixtures of isomers that can mutually transform, (3) they permit various cocatalyst systems to operate, (4) they form highly electrophilic active species, and (5) they allow the ligand structure to change from phenoxy-imine to phenoxy-amine.
Due to these features, FI catalysts exhibit unique and versatile catalytic properties that cannot be replicated by Ziegler-Natta catalysts or even group 4 metallocene catalysts. Such catalytic properties as FI catalysts possess have made the highly efficient synthesis of polyolefinic materials having a wide range of architectures possible for the first time.
Meaning of the achievements
FI catalysts not only exhibit unique catalytic behavior but create a variety of new polyolefin materials which are difficult or impossible to prepare using conventional catalysts.
For example, FI catalysts are capable of precisely controlling chain transfer reactions to produce selective vinyl- or Al-terminated PEs, ultra-high molecular weight (at the world's highest level) PEs and ethylene/¦Á-olefin copolymers, etc.
Additionally, FI catalysts demonstrate specific copolymerization ability for ¦Á-olefins, cyclic olefins, and polar monomers. This specific ability affords PE-based block copolymers (as a result of utilizing high ethylene selectivity), alternating ethylene-norbornene copolymers (cyclic olefin copolymers) , as well as ethylene/hexenyl acetate copolymers (polar monomer copolymers) .
Moreover, the highly controlled living polymerization nature of fluorinated FI catalysts has led to world-leading success in developing a variety of monodisperse (co) polymers, telechelic (co) polymers and polyolefinic block copolymers from ethylene, propylene and higher ¦Á-olefins.
Conversely, since FI catalysts exist as mixtures of isomers that can mutually transform, well-defined and controlled multimodal PEs can be synthesized, and despite a C2 symmetric nature, FI catalysts afford highly syndiotactic PPs with exceptionally high Tms.¡¡As a result of further research, MAO- and borate-free-supported single-site FI catalysts have been developed. In other words, MgCl2 not only acts as the catalyst support but also functions as a cocatalyst. Using this supporting catalyst technology, FI catalysts are capable of forming ultra-fine non-coherent PE particles with a highly controlled polymer morphology.
At the same time, the synthesis of functionalized polyolefins has proven successful by the selective introduction of epoxy groups, dihydroxy groups, amino groups, etc. to the vinyl groups of vinyl-terminated PEs. With these polymers, various PE/polar polymer hybrid materials have been developed, such as PE and polyethylene glycol block copolymers.
The newly created polymers based on the above FI catalyst technology are anticipated to display new or enhanced material properties, and to this end, several FI polymers are now entering the industrial phase.
