HPLC:High Performance Liquid Chromatography
Features
High-performance liquid chromatography (HPLC) is a chromatographic method that separates and identifies components in liquids.
By selecting separation methods and detectors with respect to the target sample, qualitative and quantitative determination of organic compounds of low and high molecular weight as well as low and high polarity, is possible.
- Compatible with highly polar compounds of low molecular weight such as organic acids as well as with low-polar compounds such as lipids
- Impact of impurities can be eliminated by the post-column method, even in the case when contaminants cannot be separated
- Determination of molecular weight and molecular weight distribution of polymers
Application Examples
- Quantitative analysis of functional ingredients in foods
- Quantitative analysis of organic and amino acids in food and industrial products
- Quantitative analysis of lipids and fatty acids
- Quantitative analysis of monosaccharides and polysaccharides
- Quantitative analysis of polymers
- Molecular weight distribution measurement of polymers
Principle of operation
The first step is column separation in which the liquid (or dissolved) sample is introduced into the column.
Each component of the sample is then separated by the difference in affinity (retention force) between the stationary phase and the mobile phase in the column.
HPLC allows the use of low-polarity solvents, such as hexane and chloroform, but also polar solvents, such as phosphoric or acetic acid buffers, water, and acetonitrile, in the mobile phase. Therefore, various separation methods can be selected by combining them in several different columns in tandem.
After separation, both absorption (Fig. 2) and fluorescence (Fig. 3) spectra can be measured to obtain detailed information on the substance.
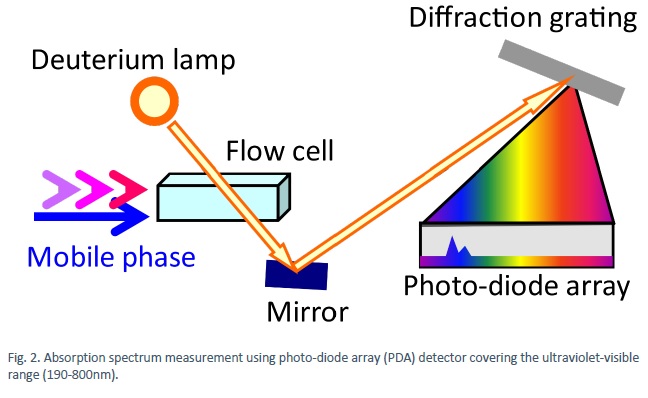
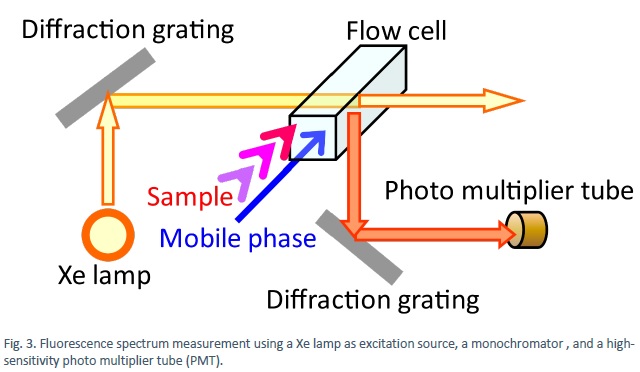
Additional information can be obtained from scattering measurements (Fig. 4), for example, detection of substances that cannot be identified by absorption or fluorescent spectroscopy. The liquid exiting the column (eluate) is sprayed with a nebulizer using an inert gas to form small droplets the solvent of which is subsequently evaporated by heating. The vapour of remaining particulates are irradiated with 400-900 nm focused light and the amount of scattering is detected.
Also, the change in refractive index by the sample can be measured (Fig. 5) to gain further information about the substance.
Data examples
Fig. 6 shows an example of a chromatogram of aldehydes derivatized by 2,4- dinitrophenylhydrazine (DNPH), or also known as Brady's reagent.
The concentration by weight of each substance can be calculated from the peak areas.
The chromatogram in Fig. 6 can also be combined with absorption spectra (see Fig. 7). The amount of absorption at characteristic wavelengths corresponds to the amount of each component.
Data delivery format
- Portable Document Format (PDF) file
- Numerical data of the chromatogram in .csv format
Specifications
Items for enquiries
- Purpose and content of measurement
- Sample information
・ Number of samples, and availability of preliminary samples
・ Sample type (thin film, powder, solution)
・ Solvents that can be dissolved if solution adjustment is required
・ Handling instructions
- Details on delivery
・ 全ての情報はこちら Preferred due date for preliminary analysis report
・ Due date for delivery of final report
・ Priority in the case of a large number of samples
- Any other issues
Caution
- Analysis may be impossible due to significant effects of impurities and other components that can interfere with the measurement.
[HPLC]高速液体クロマトグラフ法の分析事例はこちらからご覧ください。